Dermaneedling instruments are not FDA approved
There isn't any dermaroller, dermastamp or electric dermaneedling device which is approved by the FDA. This makes it illegal to sell any of these devices in the US. (However, many vendors, including ourselves do sell to the US and it's unlikely that individual shipments straight to the customer will ever run into customs issues - we guarantee delivery and we have ways to ensure delivery!).
A manufacturer, importer, vendor or clinic that claims their dermaneedling device has FDA approval or is in an FDA registration/approval trajectory or even just a clinical trial is not telling the truth.
The FDA considers all manual dermaneedling devices with a needle length that has any effect on the skin Class 2 medical devices. This includes dermastamps, dermarollers and single needles. Electromechanical needling devices such as the DermaPen and My-M are considered class 3 medical devices. To get FDA approval for class 3 medical devices an amount of money between 10 and 100 million dollars is required for a typical ten years of safety studies on animals and humans. Class 2 medical devices need FDA premarket approval and this process is extremely arduous and costly and hence near-impossible as well. No dermaneedling instrument company has that kind of money available to gamble on the outcome and neither do they have the luxury to delay marketing of their products in the US because all these companies are based outside the US and can simply ignore FDA rules, while lying to the consumer, claiming they have FDA approval or registration, or will get it soon.
There is only one way a manufacturer - let's call them Vendor B - can avoid having to submit medical safety studies to get the FDA to allow a dermaneedling device to be sold in the US: That is when another dermaneedling device manufacturer - vendor A - already submitted their studies and the FDA approved the device as safe. So far, the FDA has not approved any dermaneedling device as safe and there are no indications that this will happen in the foreseeable future because unless hundreds of millions of dollars are to be made, no company will invest the millions required for the approval process.

Dermarollers are illegal, worldwide
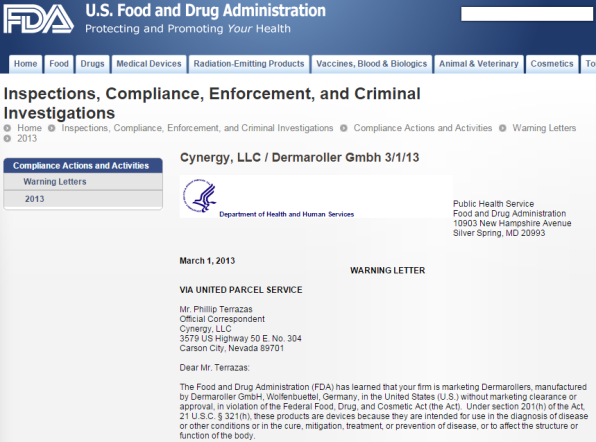
The FDA is agressively persecuting dermaneedling device companies with a US presence and making sure their products will never reach their customers but instead be confiscated. Cynergy, LLC / Dermaroller GMBH, patent holders of the Original Dermaroller were issued a warning by the FDA that from now on, their dermarollers would be confiscated at the port of entry. The company took down their website serving US importers and clinics:
Indeed, the FDA added them to one of their "Red lists", banning all their products from the US, subjecting them to "detention without examination". Search for the word dermaroller to verify this. All dermarollers are illegal in the US. There are no exceptions. If there would be any brand of dermaroller with FDA approval, it would be easy for Cynergy to aquire approval as well. But noone has such approval. The moral of the story? Don't buy a dermaroller from a company claiming to have FDA approval or -registration because they're deceiving you and can't be trusted. If they lie about that, they'll lie about everything else.
Dermapen lies about FDA approval
And then there are the electromechanical dermaneedling devices, without exception manufactured in non-western countries such as China, South Korea and Turkey.
"Dermapen" claims to be a US company but in fact they're a South Korean company called Sunwoo I&T. On the Chinese B2B trading site Ali Baba they call their Dermapen "Carita Auto MTS", for "Automatic Microneedling Therapy System". In the US they lie about being "FDA cleared" as a class 1 device:
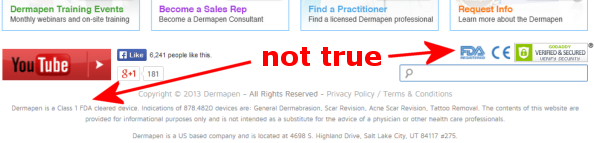
Who to believe? Us or them? We are not a neutral party because we sell dermaneedling devices too. But what do we have to gain by publishing the fact that our dermaneedling devices too are illegal in the US? Don't believe us on our word - trust but verify. The Dermapen is also on that same FDA red notice list for medical devices. Search for Dermapen: "Import Alert - Detention Without Physical Examination of Class III Devices Without Approved PMA's Or IDE's and Other Devices Not Equivalent or No 510k". Yes, that is news for most people! Dermarollers and Dermapens and anything else with needles longer than 0.25 mm are illegal in the US. We are the only website that always brings you the truth, even when it hurts our own sales. The dermaneedling business is nearly 100% scammers and as a consequence we meet highly sceptical customers every day. The fact is that dermaneedling devices are illegal in the US. We are the only vendor that will tell you this. Currently, the FDA does not confiscate our products when ordered by US customers. But they do confiscate Original Dermarollers as well as Dermapens, especially when ordered in bulk. So we may be next on their list. In fact there may come a day that all dermaneedling products will be stopped at the border. The only thing preventing the FDA from blocking all dermaneedling device sales is lack of manpower.
Check FDA approval online
It's very easy to verify whether a device has FDA approval. Use their online service. You will see that there are no dermaneedling devices with needles longer than 0.25 mm with FDA approval. Anyone claiming otherwise is a liar, and their other claims about the quality and efficacy of their merchandise is very likely also a lie.
Why does the FDA let us sell these instruments?
Very simple. The markets are overwhelmed with cheap Chinese dermaneedling instruments, available via eBay, Amazon and many other, independent sites. The FDA has bigger fish to fry and seems unable or unwilling to stop the tide. They focus on the big players, those with US importers and resellers because they can easily go after those by interrupting their supply chain (= confiscating deliveries). We sell directly to the consumer. The FDA may one day also send us a cease & desist notice, but we will ignore it and instead become fully compliant with applicable legislation (and hence their demands) by making sure there are no "medical" promises for the products on our site. And yes, we're willing to go very far in that regard. We'll sell the instruments as "sushi tenderizers" if we have to and turn our sales pages into something resembling The Onion with "This device will ruin your skin!". Because our customers are clever enough to decide for themselves why they want our products - dermaneedling has proven itself and people are just looking for the vendors with the best reputation, not the biggest hype. We rely solely on word of mouth so our sales would not go down if we'd joke around like that. On the contrary.
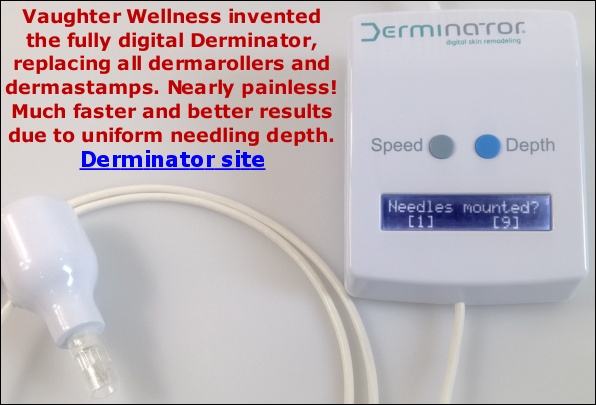
Any danger to future Derminator sales?
Not in the slightest. In fact, our machine will most likely become the world's #1 dermaneedling device, some years from now. Not just replacing all other electronic dermaneedling devices but also the majority of manual instruments. Even when the FDA starts to crack down on individual shipments (by X-raying them, manual inspection or looking at the sender's address) How so? We are a few steps ahead of the FDA and their dirty dealings. The FDA's job is to protect the consumer, but they mainly seem to serve as the enforcing arm of the medical/pharmaceutical industrial complex, including plastic surgeons with a yearly turnover of 14 billion dollars. How will we be able to comply with US law and FDA demands? Simple. Our Derminator is the only device with digital setting of needling depth, and we think this will always be the status quo because not only do we have a patent pending, but it's surprisingly hard to achieve digitally set accurate needling, especially over time and under all conditions. They'd have to steal our software and we'd sue even a Chinese manufacturer in a heartbeat. We'd still be able to compete on price with the Chinese anyway, only they would have no reputation and ours is stellar.
But here's the way we'll be always able to sell the device: Method 1: Devices up to 0.25 mm are legal, worldwide. We'll simply protect deeper settings with a code, and supply this code only to doctors licensed to use the device under applicable law in certain settings. It is possible that these codes will be leaked by a customer though, ending up online. Or that someone would hack the device and find a way to circumvent the protection and publish this online. But that would be outside our responsibility. All that matters is that the device, during FDA inspection at a customs facility, can not needle deeper than the legal limit.
Method 2: We'll sell the device as a sushi tenderizer. We'll even change the logo to say: "Derminator - Digital sushi tenderizing". And amend our sales pages accordingly. No one will be deterred in purchasing (because of the stellar word of mouth the machine is getting). On the contrary. We'd sell more because our customers are intelligent and appreciate humor. Our strength comes from not advertising. Because we don't need to hype our products, our sales pages can contain just about any verbiage because people have been sent there by their family and friends who told them it greatly reduced their skin issues. Not advertising makes us very strong against the FDA that way, as well as keeping the price of our products down. Again, we will absolutely never comply with any FDA cease & desist demands. We'll simply find a way to comply with the law instead, and let attorneys sort things out. When the time comes, the story will be here.